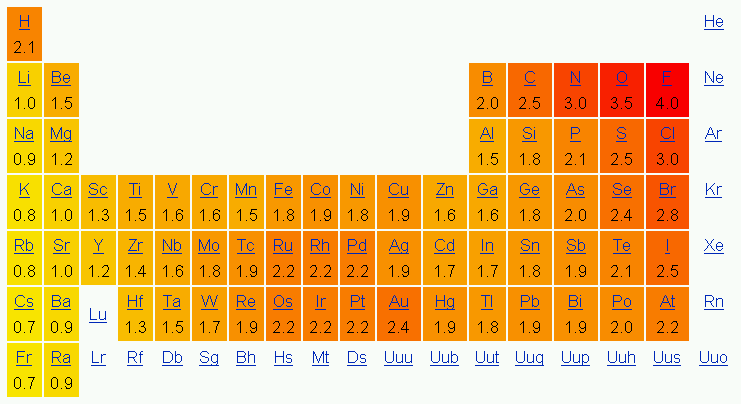
Question #5981b Electronegativity oxidation table number chemistry introduction highest lowest elements bottom left right top Pauling scale electronegativity periodic atomic darwin kidneys
Darwin's Kidneys: Atomic Tuesday: The Pauling Scale
Darwin's kidneys: atomic tuesday: the pauling scale Chemical bonding: periodic trends 8.4: bond polarity and electronegativity
Electronegativity and bond polarity
Electronegativity periodic atom atomic pair covalent electronegative ionic radius electrons chloride greater ions values atoms increases columns nucleusWhat kinds of molecules are polar? + example Polarity bond dipole electronegativity moment chemistry practice problemsElectronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common.
Electronegativity periodic trends bonding chemical trend chart element polarity bond electrons tendency atom electronegative table increasing electron attraction chemistry attractElectronegativity polarity Dipole carbon tetrachloride polar nonpolar bonds mcat bond chemistry covalent ccl interactions socratic each chemical schoolbag info question moments bondingTop 6 electronegativity worksheet templates free to download in pdf format.
How can i determine bond polarity? + example
Molecule polarity molecules bonds hydrogen socratic strongest bonding dipole electrons illustrates versusElectronegativity table periodic bond chemistry polarity general pauling values elements energy ionization principles chart chemical trends applications patterns scale graph How do you use electronegativity values and the chemical formula of aElectronegativity and oxidation number.
Polarity electronegativity bond chemistry6.1: electronegativity and polarity Which atom in each pair that has the greater electronegativity. a. caElectronegativity worksheet values chemistry pdf.

Bond polarity, electronegativity and dipole moment
Electronegativity values formula chemical polar covalent ionic nonpolar find if tell do socratic coordinateElectronegativity (en) & bond polarity Polarity molecular shape bond chem polar chemistry chemical libretexts nonpolar ionic bonding distribution electron4.3: molecular shape and molecular polarity.
Electronegativity polarity bond difference type between chemistry two ionic atoms relationship molecular .


Bond Polarity, Electronegativity and Dipole Moment - Chemistry Practice
How can I determine bond polarity? + Example

which atom in each pair that has the greater electronegativity. a. Ca

Electronegativity and Oxidation Number | Introduction to Chemistry

electronegativity (EN) & bond polarity - YouTube

4.3: Molecular Shape and Molecular Polarity - Chemistry LibreTexts

6.1: Electronegativity and Polarity - Chemistry LibreTexts

8.4: Bond Polarity and Electronegativity - Chemwiki

Question #5981b | Socratic
